Environmental DNA (eDNA) is the genetic material found in the environment. Organisms release DNA through bodily functions (secretions, mucous (slime), feces, urine, skin shed), mating processes (gametes) and death (carcasses).
These substances, and the DNA within, slowly degrade in the environment but can be collected in water or soil if caught soon enough. In the aquatic environment, eDNA is diluted, distributed by hydrologic processes and begins to degrade immediately yet eDNA can last for hours or days if the conditions are favorable, eDNA in soil can last even longer.
We use eDNA testing to monitoring for the presence or absence of native and invasive species. Species can be detected by filtering water or collecting sediment in the field, then processing the samples in the laboratory.
DNA is extracted from the water/soil samples and then identified to species using genetic markers. We offer two types of eDNA analysis: single species detection (qPCR) and community assemblage identification (DNA barcoding).
The sampling design depends on the probability of detection (PoD) and study goals.
One constant for all study designs is the need for replicate samples (a minimum of 3 per site recommended) and at least 1 field blank/sample day.
Please contact us prior to designing your experiment for more information.
For example, when detecting the presence or absence of a rare fish species in a lotic system, sample (filter) collection is recommended every 200 to 500 meters, with the distance depending upon water flow, and target species size and abundance.
The amount of DNA in the water is strongly correlated with biomass, with an adult fish releasing more DNA than a juvenile. The timing of sample collection is also important, since DNA breaks down, becomes diluted, or is transported away, so seasonal sampling may be needed to determine if species is present at different times of the year.
Like all field methods, eDNA detection will have limitations and uncertainties. Replicate samples are required to estimate presence or absence and help account for uncertainty.
The probability of detecting your target species depends on many factors, including environmental, biological, and experimental. While environmental and biological factors cannot be changed, experimental factors can be adjusted in sampling design to increase the PoD.
Please contact us to learn more about creating sampling strategy to increase you PoD by sample replication (how many filters are collected), sampling intervals (spatial and temporal) and sample volume (volume passed through the filter).
Typically, a peristaltic pump is used to draw water (up to 2L) through a filter apparatus which collects DNA. We recommend 0.45uM Sterivex filters, which are provided as part of our eDNA service.
We also accept other filter types upon client request. Strict protocols must be followed to assure that samples are not contaminated with exogenous DNA.
For example, if eDNA from a species of interest is on the sampler’s boots, hands or sampling equipment, it could contaminate the sample resulting in a positive detection when, in fact, the species is not present. Because of this, field controls must be collected to assure field crews are collecting samples properly.
QPCR is the amplification of target DNA using PCR primers and a fluorescently labeled probe. In qPCR, fluorescently labeled copies (amplicons) of target DNA are synthesized from template DNA. The fluorescent signal produced after each cycle is measured and quantified in real-time, allowing one to infer the starting quantity of the amplified DNA product. We use this technique for single species presence/absence detection.
Meta-barcoding of eDNA is a technique that allows for community level identification of taxa via PCR and high-throughput Next Generation DNA sequencing (NGS). By targeting regions of mitochondrial DNA that are shared across multiple taxa, yet variable enough to differentiate between taxa, multispecies identification is possible within a single sequencing run.
Species are grouped into taxonomic units based on DNA similarity to reference databases. Metabarcoding is dependent on the completeness and accuracy of the reference database. If references to target species do not exist or the references included in the database are incomplete or in accurate the resulting analysis will be incomplete.
qPCR is used for single species detection, and as such, qPCR assays are designed to be very specific. Metabarcoding is less specific but allows for the detection of entire groups of organisms (i.e. animals, plants, fungi, bacteria).
Costs of eDNA processing depend upon
1) the number of species one wants to detect,
2) whether a species-specific test exists for the species of interest, and
3) the number of samples to be processed.
If one wants to screen for a community of organisms in a water sample (potentially 100s), next-generation sequencing can be used, however, it can be more costly. Each project is unique and exact processing costs cannot be calculated without a discussion about your project. Contact Us
Sampling supplies are part of the cost and include filter, end-caps, adapters and tubing. Additional sampling kit equipment (pump, drill) can be borrowed for no-cost, but most clients prefer to own these items. New clients are encouraged to schedule a training session prior to designing their study.
Returning clients should contact us several weeks prior to the project start date to ensure sampling supply availability.
Per sample cost depends upon test type:
Single species detection (qPCR). qPCR is cost-effective for small sample projects and/or for the detection of 1-7 species.
Community Assemblage (DNA barcoding). DNA barcoding is best for larger projects, identification of 5 or more species, or when target species is not specified or known.
Cost of eDNA processing is per sample and depends upon
1) number of species requested and
2) species-specific assay availability.
If the species of interest is on our assay list, we offer cost for a single sample (filter) analyzed for a single species. Additional species can be added at significantly reduced cost. For species not yet on our list, we offer custom qPCR development. Contact us for current pricing.
Screening eDNA to identify species within a community of organisms requires a next-generation sequencing approach. This process allows for the simultaneous testing of multiple samples with a single test and is a fixed cost for 1-50 samples. Currently, we offer this service for only fish community assessment but can implement DNA barcode sequencing for other taxonomic groups upon request. Contact us for current pricing and additional details.
If there is not a species-specific qPCR assay already developed for your target species, we can develop one for you. See Our Assay List The cost for a qPCR assay will vary depending on how much information is publicly available. Assay design follows two potential processes:
Regardless of the assay development process, for some species, there are regional differences between populations, so reference samples are required from your study area. Reference samples (positive controls) contain the genetic information specific to your study area and are required for assay design and development. We can assist in the process of locating reference samples if necessary. See Our Assay List
Processing consists of DNA extraction, qPCR or metabarcoding analysis, data analysis, and reporting. Processing time for common species for which qPCR assays are already available takes only hours however actual turnaround time will depend on project size and current laboratory queue. See Our Assay List
We do offer “Rapid” testing for an extra cost. Rapid is for 12 or fewer samples and has a maximum return time of 48hrs (contact us if Rapid is needed for larger projects – we may be able to accommodate your request).
If a qPCR assay does not exist and one must be designed and validated processing times will need to be discussed in greater detail. If you need an assay designed and validated, please contact us directly to discuss your project.
The sampling design depends on the probability of detection and goals of the study. For detecting presence or absence of rare species, samples are recommended to be collected every 5 to 500 meters, though the distance depends upon flow and the size and abundance of particular species. The amount of DNA in the water is strongly correlated with biomass, so an adult salmon would release more DNA into the water than a juvenile salmon. The timing of sample collection is also important as the DNA starts to break down immediately and becomes diluted so seasonal samples may be needed to determine if species are present at different times of the year.
Like most field methods, eDNA detection will have limitations and uncertainties. Replicate samples are required to estimate presence or absence and account for uncertainty. The probability of detecting your target species is the percent chance that you will capture target species DNA within your filtered sample and subsequent analysis. There are many factors that influence the PoD, however there are only a few that can be controlled, namely sample replication (how many filters are used?), sampling intervals (spatial and temporal), filter type and pore size, DNA extraction technique, analysis method/chemistry, technical replication, and data analysis/interpretation.
Estimating the number of individuals that are present in an aquatic, therefore mixed, ecosystem based on the amount of eDNA detected is very difficult. We feel that the technology required to provide accurate correlations between the amount of eDNA detected and biomass in an open, flowing, aquatic environment is not available. It is possible to compare the amount of eDNA detected between sites and over time to infer occupancy and relative density. It is also possible to infer the numbers of individuals from the amount of eDNA detected from certain types of terrestrial samples as these samples are not mixed and assumed to come from an individual. Scat or hair taken must have come from one organism thus can be counted as a sample from an individual.
eDNA is generally targeting a kind of DNA (mitochondrial) that does not provide direct evidence of hybridization, as mitochondria are inherited from the mother.
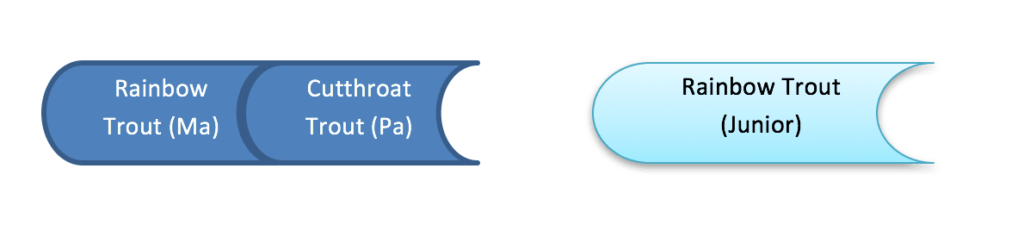
eDNA could provide some insight into whether hybridization could potentially occur, by verifying that both species of concern are present at the same location.
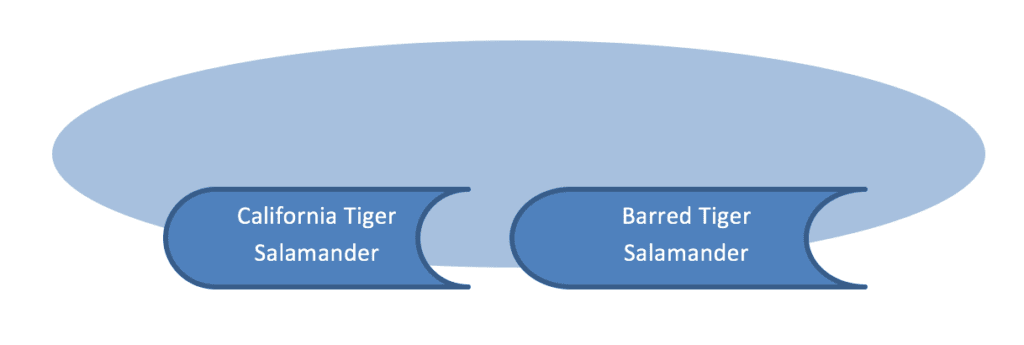
Hybridization is the occurrence of reproduction between two species. In other words, a hybrid individual would show a mixture of genetic characteristics from both parental species.
Three pieces of information are required to observe hybridization:
In the above example, offspring that arise from a reproductive event between two different species would appear 50% species A and 50% species B (on average, across many locations in the genome). Genetic patterns could get quite complicated though if hybrid offspring persisted (and reproduced over time) or hybrid individuals reproduced with original parental species. Nevertheless, the point here is that a hybrid is a blend of source species and appears at the genotype level to share DNA sequences from both species.
Genetic introgression is the movement of genes from one species into another species via persistent and ongoing hybridization events.
Genidaqs
+1 916-231-1681 / genidaqs@fishsciences.com
3300 Industrial Blvd., Suite 100
West Sacramento, CA 95691
Cramer Fish Sciences
©2026
"*" indicates required fields